Слайд 1Environmental Administration and Legislation
Mikkeli University of Applied Sciences Autumn 2016
Слайд 2REVISION What did you learn last week?
18.10.2016
Environmental Administration and Legislation, 2016
Слайд 3EU waste legislation
Framework legislation on waste Legislation on waste management operations Legislation on specific waste streams Reporting legislation
Слайд 4Directive on waste: Waste hierarchy*
“In order to better protect the environment, the Member States should take measures for the treatment of their waste in line with the following hierarchy which is listed in order of priority: prevention; preparing for reuse; recycling; other recovery, notably energy recovery; disposal.”
Слайд 5Directive on waste: Permits and Registrations
Any establishment or undertaking intending to carry out waste treatment must obtain a permit (IPPC licence) from the competent authorities who determine notably the quantity and type of treated waste, the method used as well as monitoring and control operations.
Слайд 6Treatment Operations
Prevention Preparing for reuse Recycling Tax incentives, bans, … other recovery, notably energy recovery Incineration Disposal Landfill
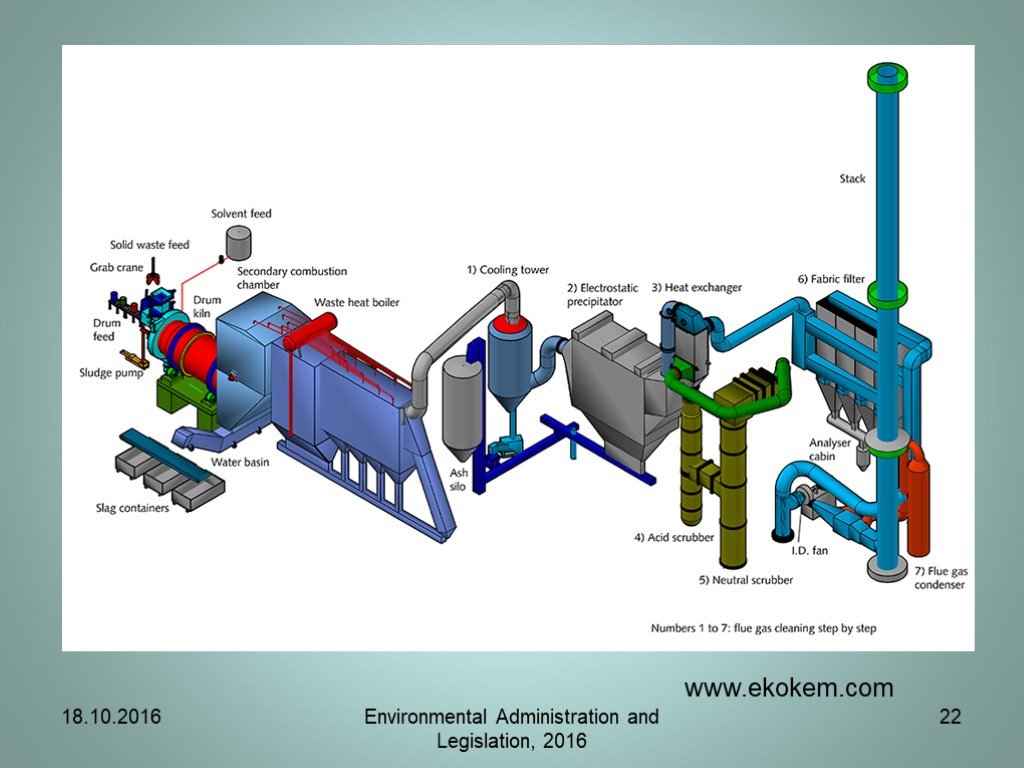
Слайд 7Incineration of waste
The WI Directive sets emission limit values and monitoring requirements for pollutants to air such as dust, nitrogen oxides (NOx), sulphur dioxide (SO2), hydrogen chloride (HCl), hydrogen fluoride (HF), heavy metals and dioxins and furans. The Directive also sets controls on releases to water resulting from the treatment of the waste gases. Most types of waste incineration plants fall within the scope of the WI.
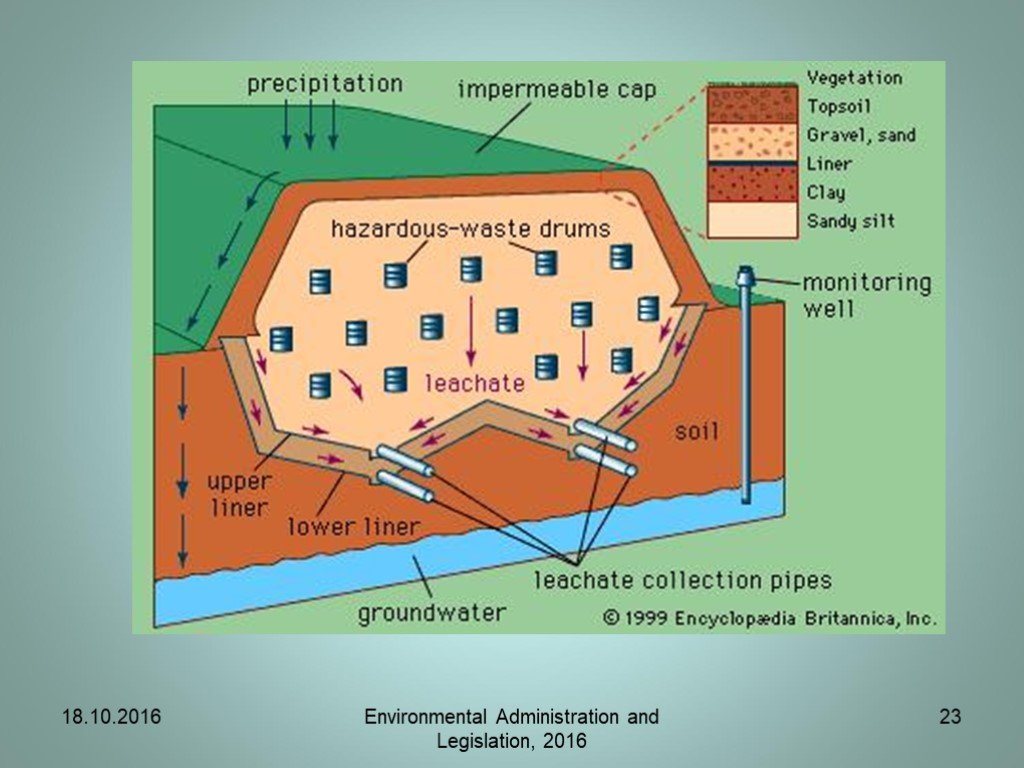
Слайд 8Landfill of waste
The Landfill Directive applies to all landfills, defined as waste disposal sites for the deposit of waste onto or into land. Defines the different categories of waste: municipal waste, hazardous waste, non-hazardous waste and inert waste (waste which is neither chemically or biologically reactive and will not decompose)
Слайд 9Hazardous Waste Waste from Consumer Goods Packaging Waste Waste from Specific Activities Radioactive Waste and Substances
Слайд 10Directive 2006/66/EC on batteries and accumulators and waste batteries and accumulators (and amending acts).
“The producers have to bear the cost of collecting, treating and recycling industrial, automotive and portable batteries and accumulators, as well as the costs of campaigns to inform the public of these arrangements. Small producers may be exempted from this obligation if this does not impede the proper functioning of the collection and recycling schemes. All producers of batteries and accumulators have to be registered.”

Слайд 11Directive 2012/19/EU on waste electrical and electronic equipment (WEEE)
Designed to prevent electrical and electronic waste by requiring EU countries to ensure the equipment is recovered, reused or recycled. Producers have to make a financial contribution to cover the costs of collecting, treating and sustainably disposing of both non-household equipment and private electrical waste deposited at dedicated collection points. Variable collection target from 2016 onwards, taking account of individual national economies: 45 % of the average weight of products placed on the market in a given country in the 3 preceding years.

Слайд 12Legislation on Packaging and Packaging Waste
Directive 94/62/EC on packaging and packaging waste covers all packaging placed on the European market and all packaging waste, whether it is used or released at industrial, commercial, office, shop, service, household or any other level, regardless of the material used. Member States should take measures to prevent the formation of packaging waste, and to develop packaging reuse systems reducing their impact on the environment. Specific targets for packaging waste recovery or incineration and reduction of materials contained in packaging waste must be attained.
Слайд 13Legislation Controlling Hazardous Waste Management
Слайд 14Hazardous Waste
Hazardous wastes pose a greater risk to the environment and human health than non hazardous wastes and thus require a stricter control regime.
Слайд 15Directive 2008/98/EC provides additional labelling, record keeping, monitoring and control obligations from the "cradle to the grave” Mixing of hazardous substances is banned in order to prevent risks for the environment and human health. The permit exemptions that may be granted to installations dealing with hazardous wastes are more restrictive than for installations dealing with other wastes.
Слайд 16Convention on the control of transboundary movements of hazardous wastes and their disposal (Basel Convention) 1997 Council Directive (91/689/EEC) on hazardous waste -> repealed by Directive 2008/98/EC (Directive on waste) Decision 2000/532/EC List of Hazardous Wastes: Definition of hazardous waste Harmonised list of wastes
Слайд 17The classification into hazardous and non hazardous waste is based on the system for the classification and labelling of dangerous substances and preparations, which ensures the application of similar principles over their whole life cycle. The properties which render waste hazardous are laid down in Annex III of Directive 2008/98/EC and are further specified by the Decision 2000/532/EC establishing a List of Wastes as last amended by Decision 2001/573/EC. (The List of Wastes is currently being reviewed)
Слайд 18Wastes classified as hazardous are considered to display one or more of the properties listed in Annex III to Directive 91/689/EEC and, as regards H3 to H8, H10 (1 ) and H11 of the said Annex, one or more of the following characteristics: — flash point ≤ 55 ºC, — one or more substances classified (2 ) as very toxic at a total concentration ≥ 0,1 %, — one or more substances classified as toxic at a total concentration ≥ 3 %, ….
Слайд 19List includes 20 categories of waste, each containing several subcategories. 01 Wastes resulting from exploration, mining, quarrying, physical and chemical treatment of minerals 02 Wastes from agriculture, horticulture, aquaculture, forestry, hunting and fishing, food preparation and processing ….
Слайд 20Hazardous Waste Management
Collection – requirements for registers, containers and transportation. Treatment – requirements on treatment facilities. Disposal – requirements for HW landfills Hazardous wastes can take the form of solids, liquids, sludges, or contained gases. This needs to be considered when deciding on the management options.
Слайд 21
Слайд 23
Слайд 24Legislation Controlling the Use of Hazardous Substances
Слайд 25REACH
“The EU has modernized European chemicals legislation and established an integrated system for the registration, evaluation, authorisation and restriction of chemicals.” objective is to improve the protection of human health and the environment European Chemicals Agency
Слайд 26The idea is that “testing on humans” is no longer accepted – the burden of proving that chemicals produced and placed on the market are safe is now on the chemicals industry. (Precautionary Principle) “REACH requires firms which manufacture and import chemicals to evaluate the risks resulting from the use of those chemicals and to take the necessary steps to manage any identified risk.”
Слайд 27Risk Management
Management of a risk requires steps of Hazard identification Assessment of the risk(s) caused by this hazard (including likelihood and severity of effects) Deciding on risk control option (avoiding, mitigating, transfering or accepting the risk) Monitoring
Слайд 28REACH: Scope
The scope of the Regulation covers all substances , whether manufactured, imported, placed on the market, or used on their own or in mixtures with the exceptions of: radioactive substances (covered by Directive 96/29/Euratom); substances under customs supervision which are in temporary storage, in free zones or free warehouses with a view to re-exportation or still in transit; non-isolated intermediates ; the transport of dangerous substances; and waste.
Слайд 29The rules on registration, downstream users, evaluation and authorisation do not apply to substances used in medicinal products for human or veterinary use or in food or feedingstuffs (including additives) provided they fall within the scope of Community legislation on medicinal products or food.
Слайд 30Registration, Evaluation, Authorisation and restriction of CHemicals.
Слайд 31REACH: Registration
Registration is the key component of the REACH system. It is compulsory to register in a central database chemicals which are manufactured or imported in quantities of one tonne or more per annum. The database is managed by the European Chemicals Agency. If a substance is not registered it cannot be produced or placed on the European market.
Слайд 32Some groups of substances (listed in the Regulation) are exempt from the obligation to register, for example: polymers (however monomers which make up polymers must still be registered); some substances for which the estimated risk is negligible (water, glucose, etc.); naturally occurring and chemically unaltered substances; substances used in research and development, under certain conditions.
Слайд 33Registration requires the industry to provide information on the properties and uses of chemicals and the precautionary measures to be taken when using them. The data required are proportional to the production volume of and the risks presented by the substance concerned (for example, extensive toxicity tests for substances manufactured or imported in quantities of more than 1000 tonnes).
Слайд 34An application to register a substance which is imported or manufactured in a quantity of 10 tonnes or more per year must include a detailed description of the risks associated with that substance and the different possible exposure scenarios and risk management measures (chemical safety report).

Слайд 35There are special arrangements for the registration of substances present in articles: given the millions of articles that are placed on the market in the EU and the potential risk some of them represent to human health and the environment, certain substances incorporated into articles must be registered. Registration is compulsory when the substance in question is normally released when the article is used and is present in those articles in quantities totalling over one tonne per producer or importer per year. For substances that are not normally released but which are particularly hazardous and are contained in a minimum concentration of 0.1% and placed on the market in quantities of over one tonne per producer or importer per year, simple notification is required, on the basis of which the European Chemicals Agency may request a registration.

Слайд 36The European Chemicals Agency is responsible for managing the database, receiving registration dossiers and developing technical guides aimed at helping manufacturers, importers and the competent authorities in implementing these provisions. During the first eleven years of application of the REACH system, around 30 000 substances already on the market should be registered.
Слайд 37Downstream users must consider the safety of substances, based primarily on information from their suppliers, and to take appropriate risk management measures. These provisions also allow authorities to have an overview of the uses of a substance as it moves through the supply chain and, if necessary, to request further information and take appropriate measures.
Слайд 38
Слайд 39REACH: Evaluation
Evaluation makes it possible for the Agency to check that industry is fulfilling its obligations and avoiding tests on vertebrate animals when unnecessary. Two types of evaluation are provided for: dossier evaluation substance evaluation
Слайд 40Dossier evaluation is compulsory for any applications to carry out tests specified in Annexes IX and X to the Regulation (these are the most stringent tests, mostly involving the use of vertebrate animals). The aim is essentially to minimise the need for experiments of this kind. Dossier evaluation may also be carried out in order to check the conformity of a registration. The Agency is expected to carry out a thorough review of at least 5% of the dossiers filed.
Слайд 41Substances suspected of posing a risk to human health or the environment may also be evaluated by the competent authorities in the Member States in order to determine whether further information is required. The evaluation programme is developed by the Agency, in cooperation with the competent authorities.
Слайд 42If a substance is suspected of posing a risk to human health or the environment, the Agency will include this substance in a specific list and a designated Member State will carry out an evaluation in order to determine whether further information is required from the registrant.
Слайд 43Evaluation can lead to the following conclusions: the substance must be subject to restriction or authorisation procedures; the classification and labelling of the substance must be harmonised; ….
Слайд 44… information must be supplied to the other authorities so that they can adopt appropriate measures. For example, if, while the substance is being evaluated, information on risk management measures become available and could have an impact on the conditions of use of that substance, the information should be transmitted to the authorities responsible for this legislation.
Слайд 45
Слайд 46REACH: Authorisation
Substances of extremely high concern may be subject to authorisation by the Commission with regard to particular uses. The objective is to ensure that the risks linked with these substances are validly controlled and that these substances are gradually replaced by other appropriate substances or technologies where this is economically and technically viable.
Слайд 47The Agency publishes and regularly updates a list of substances (list of candidate substances) identified as having characteristics of extremely high concern. These may include the following: CMRs (carcinogens, mutagens and reproductive toxins); PBTs (persistent, bioaccumulative and toxic substances); vPvBs (very persistent and very bioaccumulative substances); some substances of concern which have irreversible serious effects on humans and the environment, such as endocrine disruptors.

Слайд 48After inclusion of this substance any placing on the market and use of such chemical substances is subject to authorisation. This is granted if the risks arising from the substance in question can be validly controlled. If they cannot and if no alternative exists, the Commission is to assess the level of risk and the socio-economic advantages of using the substance and decide whether to authorise it or not. Some substances, such as PBTs and vPvBs can be authorised only if the socio-economic advantages override the risks and there are no alternatives.
Слайд 49The burden of proof is placed on the applicant. All authorisations must be reviewed after a certain period of time, determined on a case-by-case basis. Downstream users may use a substance for an authorised use provided they obtain the substance from a company to which an authorisation has been granted and keep within the conditions of that authorisation.
Слайд 50Registration, Evaluation, Authorisation and Restriction of CHemicals.
Слайд 51REACH: Restrictions
The restriction procedure provides a safety net, making it possible to manage the risks which are not adequately covered by other provisions of the REACH system.
Слайд 52may relate to the conditions of manufacture, use(s) placing on the market of a substance, or the possible prohibition of such activities, if necessary.
Слайд 53Restrictions are suggested by Member States or by the Agency and decided on by the Commission. Please visit: echa.europa.eu (link in Moodle) for further information
Слайд 54ECHA
The Regulation establishes a European Chemicals Agency, responsible for managing the technical, scientific and administrative aspects of REACH and ensuring consistency of decision-making at Community level. The Agency is also to manage the registration process and play a key role in the evaluation process. It receives applications for authorisation and delivers opinions and issues recommendations in relation to the authorisation and restriction procedures.
Слайд 55Competent Authorities
The Regulation requires there to be authorities in each of the Member States with the competence and resources necessary to carry out the tasks assigned to them. These authorities must cooperate with each other and with the Agency in the performance of their duties.
Слайд 56Competent Authorities: Finland
The following authorities are responsible for the enforcement of REACH Regulation in Finland: The Ministry of Social Affairs and Health and the Ministry of the Environment are responsible for the overall management and supervision of the REACH Regulation in Finland. The Finnish Safety and Chemicals Agency (Tukes) has been appointed as the REACH Competent Authority and is also responsible for REACH enforcement in regard to all provisions relating to placing on the market of chemicals. Tukes is responsible for the national REACH Helpdesk.
Слайд 57The CLP Regulation
Слайд 58The classification, labelling and packaging of dangerous substances have been harmonised since 1967 to ensure the protection of health and the environment, and to ensure the free movement of such products. Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures (amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006.)

Слайд 59The regulation seeks to ensure that European Union workers and consumers are clearly informed of the hazards associated with chemicals by means of a system of classification and labelling. The aim is to ensure that the same hazards are described and labelled in the same way in all EU countries. It lays down uniform requirements for the classification, labelling and packaging of chemical substances and mixtures according to the United Nations’ Globally Harmonized System (GHS). It requires companies to classify, label and package appropriately their hazardous chemicals before placing them on the market.

Слайд 60CLP Pictograms
A hazard pictogram is an image on a label that includes a warning symbol and specific colours intended to provide information about the damage a particular substance or mixture can cause to our health or the environment. The new pictograms are in the shape of a red diamond with a white background, and will replace the old orange square symbols which applied under the previous legislation.
Слайд 61Please visit: echa.europa.eu (link in Moodle) for further information.